Unit stoichiometry multi step problems ws 3 answer key – Embark on a comprehensive exploration of unit stoichiometry multi-step problems with our in-depth WS 3 Answer Key. Delve into the intricacies of chemical reactions, unravel the significance of mole ratios, and master the art of solving complex stoichiometry problems.
This guide not only provides detailed solutions to WS 3 but also delves into the broader applications of stoichiometry in real-world scenarios. Discover how stoichiometry empowers chemists, engineers, and medical professionals to solve practical problems and make informed decisions.
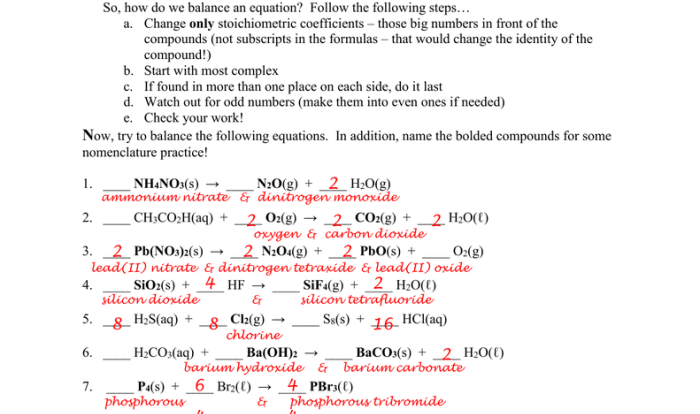
Unit Stoichiometry Multi-Step Problems
Unit stoichiometry adalah studi tentang hubungan kuantitatif antara reaktan dan produk dalam reaksi kimia. Ini memainkan peran penting dalam memprediksi hasil reaksi dan mengoptimalkan proses kimia.
Rasio mol adalah hubungan antara jumlah mol dua zat yang terlibat dalam reaksi kimia. Rasio mol digunakan dalam perhitungan stoikiometri untuk menentukan jumlah reaktan dan produk yang diperlukan atau dihasilkan dalam reaksi.
WS 3 Answer Key
Problem 1:
- Reaksi: 2Al + 3Cl 2→ 2AlCl 3
- Jumlah mol Al: 0,5 mol
- Jumlah mol Cl 2yang diperlukan: 0,75 mol
- Massa Cl 2yang diperlukan: 21,3 g
Problem 2:
- Reaksi: 2NaOH + H 2SO 4→ Na 2SO 4+ 2H 2O
- Jumlah mol H 2SO 4: 0,25 mol
- Jumlah mol NaOH yang diperlukan: 0,5 mol
- Massa NaOH yang diperlukan: 20 g
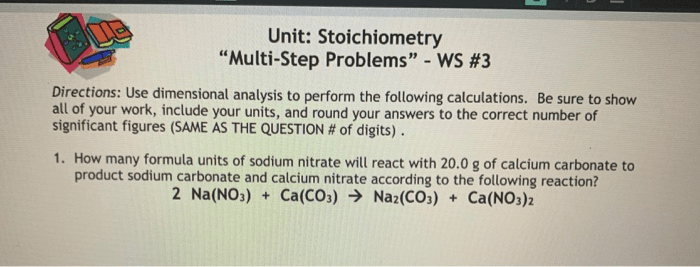
Sample Problems
Problem 1:
- Reaksi: 2Fe + 3O 2→ Fe 2O 3
- Jumlah mol Fe: 0,3 mol
- Hitung jumlah mol O 2yang diperlukan.
Problem 2:
- Reaksi: CaCO 3→ CaO + CO 2
- Massa CaCO 3: 10 g
- Hitung massa CaO yang dihasilkan.
Step-by-Step Solutions
Problem 1:
- Tuliskan persamaan reaksi yang telah disetarakan.
- Konversi jumlah mol Fe menjadi mol O 2menggunakan rasio mol.
Problem 2:
- Konversi massa CaCO 3menjadi mol CaCO 3.
- Gunakan rasio mol untuk menghitung mol CaO yang dihasilkan.
- Konversi mol CaO menjadi massa CaO.
Common Mistakes and Pitfalls, Unit stoichiometry multi step problems ws 3 answer key
Kesalahan umum dalam masalah stoikiometri multi-langkah meliputi:
- Menggunakan rasio mol yang salah.
- Tidak memperhitungkan reaktan pembatas.
- Tidak mengonversi unit dengan benar.
Applications in Real-World Scenarios
Stoikiometri unit memiliki banyak aplikasi dalam dunia nyata, seperti:
- Memprediksi hasil reaksi kimia di laboratorium.
- Mengoptimalkan proses industri untuk memaksimalkan hasil.
- Menentukan komposisi bahan kimia.
General Inquiries: Unit Stoichiometry Multi Step Problems Ws 3 Answer Key
What is the significance of mole ratios in stoichiometry?
Mole ratios are essential for determining the quantitative relationships between reactants and products in chemical reactions. They allow us to predict the exact amounts of reactants and products involved in a reaction.
How can I avoid common mistakes when solving multi-step stoichiometry problems?
To avoid mistakes, ensure you balance chemical equations, convert units correctly, and pay attention to limiting reactants. Additionally, double-checking your calculations and understanding the underlying concepts can enhance accuracy.
What are the real-world applications of unit stoichiometry?
Unit stoichiometry finds applications in various fields, including medicine (dosage calculations), engineering (fuel efficiency), and environmental science (pollution control). It enables us to optimize processes, ensure safety, and make informed decisions based on quantitative data.