Chemistry the central science 13th ed – Chemistry: The Central Science, 13th Edition, invites readers to embark on a captivating journey into the realm of matter and its transformations. This comprehensive textbook provides a lucid and engaging exploration of the fundamental principles, concepts, and applications of chemistry, empowering students with a deep understanding of the subject.
From the intricacies of atomic structure to the complexities of biochemical reactions, Chemistry: The Central Science, 13th Edition, unveils the fascinating world of chemistry. It illuminates the interconnectedness of matter, energy, and change, showcasing how chemical processes shape our physical world and underpin countless technological advancements.
Core Concepts and Principles
Chemistry, the study of matter and its interactions, is founded on fundamental principles that govern the behavior of substances and their transformations. These principles include:
Matter and Energy
- Matter exists in various forms, including solids, liquids, and gases, and can undergo physical and chemical changes.
- Energy is a property of matter and can be transferred or transformed from one form to another.
Chemical Reactions
- Chemical reactions involve the rearrangement of atoms and molecules, leading to the formation of new substances.
- Chemical reactions are characterized by the breaking and formation of chemical bonds.
Conservation Laws
- Mass and energy are conserved in chemical reactions, meaning the total mass and energy of the reactants and products remain the same.
Atomic and Molecular Structure: Chemistry The Central Science 13th Ed
Atoms, Chemistry the central science 13th ed
Atoms are the fundamental building blocks of matter, consisting of a nucleus containing protons and neutrons, surrounded by electrons.
Chemical Bonding
- Atoms combine to form molecules and compounds through chemical bonding.
- Types of chemical bonds include covalent, ionic, and metallic bonds.
Molecular Structure
- The arrangement of atoms in molecules determines their shape and properties.
- Molecular polarity and intermolecular forces influence the physical and chemical behavior of molecules.
States of Matter
Solids
- Solids have a fixed shape and volume.
- Particles in solids are closely packed and have limited mobility.
Liquids
- Liquids have a fixed volume but no definite shape.
- Particles in liquids are loosely packed and can move past each other.
Gases
- Gases have neither a fixed shape nor volume.
- Particles in gases are widely dispersed and move freely.
Phase Transitions
- Phase transitions occur when a substance changes from one state of matter to another.
- Factors such as temperature and pressure influence phase transitions.
Solutions and Their Properties
Solutions
Solutions are homogeneous mixtures of two or more substances.
Types of Solutions
- Solutions can be classified based on the physical state of the components (e.g., solid-liquid, liquid-liquid).
- The concentration of a solution determines the amount of solute present in a given amount of solvent.
Solubility
- Solubility is the ability of a solute to dissolve in a solvent.
- Factors such as temperature, pressure, and molecular structure influence solubility.
Chemical Reactions and Stoichiometry
Types of Chemical Reactions
- Chemical reactions can be classified based on their characteristics, such as combination, decomposition, and redox reactions.
- Each type of reaction has unique features and applications.
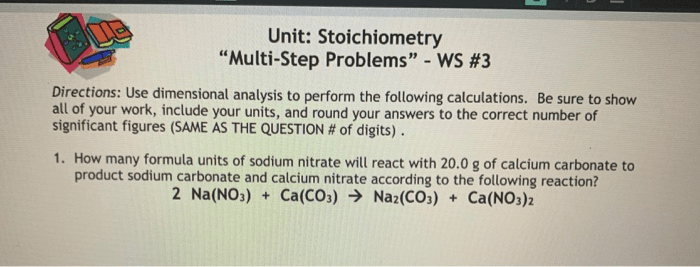
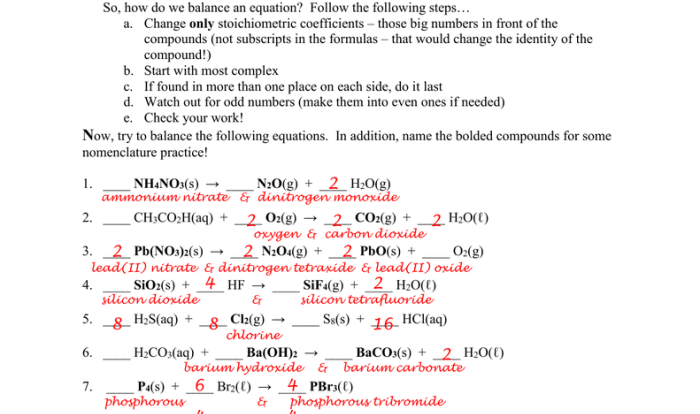
Stoichiometry
- Stoichiometry involves determining the quantitative relationships between reactants and products in a chemical reaction.
- Balanced chemical equations represent the stoichiometric ratios of reactants and products.
Thermodynamics and Chemical Equilibrium
Thermodynamics
Thermodynamics deals with the energy changes associated with chemical reactions and physical processes.
Chemical Equilibrium
- Chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction occur at the same rate.
- Factors such as temperature, pressure, and concentration affect the equilibrium position.
Acids, Bases, and pH
Acids and Bases
- Acids are substances that donate protons (H+ ions), while bases accept protons.
- The strength of acids and bases is measured on the pH scale.
pH Scale
- The pH scale is a measure of the acidity or basicity of a solution.
- Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic.
Organic Chemistry
Scope of Organic Chemistry
Organic chemistry focuses on the study of compounds containing carbon.
Structure and Properties of Organic Molecules
- Organic molecules have unique structures and properties due to the versatility of carbon.
- Functional groups play a crucial role in determining the reactivity and properties of organic compounds.
Reactions of Organic Compounds
- Organic compounds undergo a wide range of reactions, including substitution, addition, and elimination reactions.
- Understanding organic reactions is essential for various applications.
Biochemistry
Chemistry in Biological Systems
Biochemistry explores the chemical processes and molecules that occur in living organisms.
Biomolecules
- Biomolecules include carbohydrates, proteins, lipids, and nucleic acids.
- The structure and function of biomolecules are essential for understanding biological processes.
Metabolic Pathways
- Metabolic pathways are interconnected chemical reactions that occur in cells.
- Understanding metabolic pathways provides insights into cellular processes and health.
Applications of Chemistry

Chemistry in Medicine
- Chemistry plays a vital role in the development and production of drugs, vaccines, and medical devices.
- Understanding the chemical processes in the body aids in diagnosis and treatment of diseases.
Chemistry in Industry
- Chemistry is essential for the production of materials, fuels, and other products used in various industries.
- Chemical processes are optimized to increase efficiency and reduce environmental impact.
Chemistry in Environmental Science
- Chemistry helps us understand and address environmental issues such as pollution, climate change, and resource depletion.
- Chemical analysis and monitoring are crucial for environmental protection and sustainability.
Key Questions Answered
What is the main theme of Chemistry: The Central Science, 13th Edition?
The central theme of the textbook is the interconnectedness of matter, energy, and change, and how chemical processes shape our physical world and underpin countless technological advancements.
What are the key features of Chemistry: The Central Science, 13th Edition?
Key features include lucid explanations, captivating examples, real-world applications, interactive exercises, and a wealth of supplementary resources to enhance student learning.
Who is the intended audience for Chemistry: The Central Science, 13th Edition?
The textbook is primarily intended for undergraduate students seeking a comprehensive and engaging introduction to chemistry, whether for their major or as a supporting course for other disciplines.
How does Chemistry: The Central Science, 13th Edition, differ from other chemistry textbooks?
The textbook stands out with its emphasis on the interconnectedness of chemistry and its applications in the real world. It presents complex concepts in a clear and accessible manner, making chemistry relatable and engaging for students.
What are the benefits of using Chemistry: The Central Science, 13th Edition, in the classroom?
The textbook provides a solid foundation in chemistry, fostering a deep understanding of the subject and preparing students for further studies or careers in chemistry-related fields.